_Ά/publication
Precise synthesis of copper selenide nanowires with tailored Cu vacancies through photo-induced reduction for thermoelectric applications
Shunya Sakane, Tatsuki Miura, Kazuki Munakata, Yusuke Morikawa, Shunichiro Miwa, Riku Yanmanaka, Mayu Akutsu, Toshiki Sugai, Akito Ayukawa, Haruhiko Udono, and Hideki Tanaka
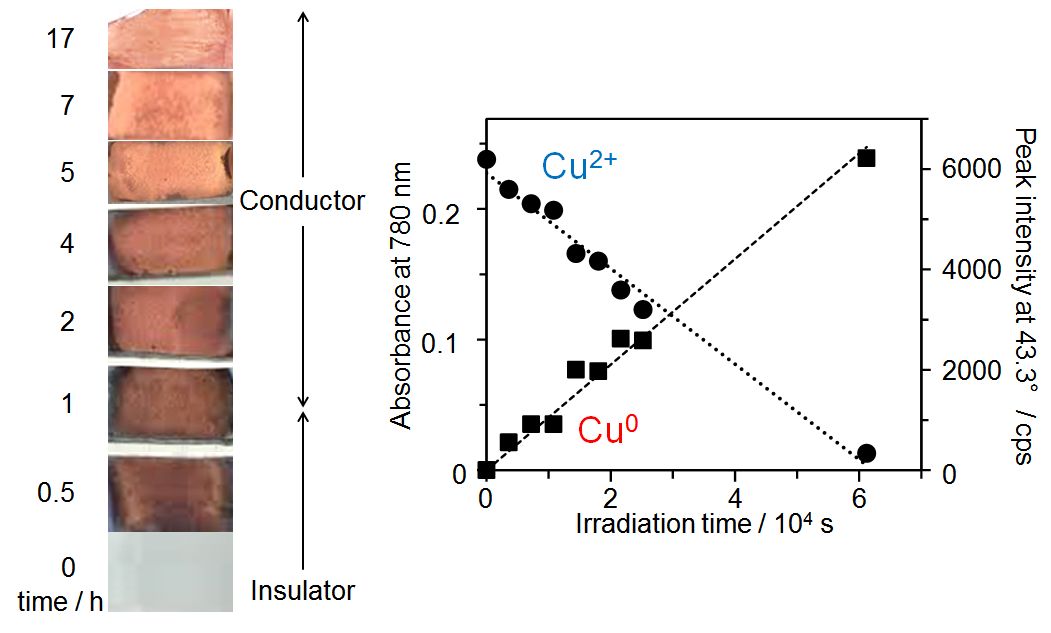
Nanostructuring in Ώ-Cu2Se while optimizing carrier concentration holds the promise of realizing further high thermoelectric performance at near room temperature. Nevertheless, controlling the amounts of Cu vacancies, which work as acceptors, in nanostructures is considerably more intricate than in bulk materials. Hence, controlling the amounts of Cu vacancies while maintaining the Ώ-phase and nanostructure shape poses a formidable challenge. In this study, we synthesized Cu2+xSe nanowires (NWs) with various amounts of Cu vacancies at room temperature by the photoreduction method and investigated their thermoelectric properties. Cu2+xSe NWs exhibited a comparable thermoelectric power factor to that of the polycrystalline films fabricated at higher temperature. The achievement of the high power factor despite low-temperature fabrication is attributed to the precise synthesis of Cu2+xSe NWs with various amounts of Cu vacancies. We also investigated the reaction process of Cu2.00Se NWs in detail by observing the reaction intermediates. It was found that photoreduction occurred with Cu2+ ions adsorbed on Se NWs, leading to the reaction of Cu2+ ions and Se NWs without Cu deficiency. Namely, this photoreduction under the adsorbed conditions realized the control of Cu vacancies in Cu2+xSe NWs.
Shunya Sakane, Kai Akimoto, Kishin Konishi, Kenta Takaoka, Harunobu Iwatsuki, Mayu Akutsu, Toshiki Sugai, and Hideki Tanaka
Cu nanoparticles (NPs) as catalysts have the good advantage of being more abundant than noble metal NPs. In this study, we synthesized nonaggregating Cu NPs supported in Y-type zeolite by the photoreduction method. In this method, Cu ions in pores of zeolite can be slowly reduced with a small amount of reductant at room temperature. The high-resolution transmission electron microscope, energy dispersive X-ray spectroscopy, X-ray diffraction patterns, and UV-Vis spectra supported that nonaggregating Cu NPs existed in t
he pores of zeolite. Catalytic activities of Cu NP-zeolite were investigated for the aerobic oxidation of benzyl alcohol. Our Cu NP-zeolite had a large turnover frequency of 17 h-1. The yield of benzaldehyde increased in proportion to the amount of Cu loading at
0.5 wt %, indicating that Cu NPs in pores of zeolite work as catalysts for selective aerobic oxidation of benzyl alcohol. The high catalytic activity was brought by nonaggregating Cu NPs in pores of zeolite. The catalytic reaction for other aromatic alcohols with electron-donating groups proceeded, whereas it did not proceed for the aromatic alcohols with electron-withdrawing groups or aliphatic alcohols, indicating that the interaction between zeolite and the benzene ring also contributed to the reaction. This study would be expected to contribute to the development of Cu NP catalysts.
Shunya Sakane, Toshiki Anji, Itsuki Yamagishi, Issei Kohara, and Hideki Tanaka
In this study, we synthesized copper nanoparticles (Cu NPs) with poly(N-isopropylacrylamide) (PNIPAM) by photoreduction at room temperature. The synthesized Cu NP/PNIPAM solution of dark red changed to cloudy red at 27 C by thermal response of PNIPAM. The thermoresponsive behavior of Cu NP/PNIPAM was also caused by visible light irradiation because Cu NPs gained thermal energy through plasmonic heating. This study would contribute to the development of the plasmonic heating using Cu NPs.
Shunya Sakane,Shunichiro Miwa, Tatsuki Miura, Kazuki Munakata, Takafumi Ishide, Yoshiaki Nakamura, and Hideki Tanaka
Organic materials have attracted attention for thermoelectric materials reusing low-temperature waste heat. For the thermoelectric performance enhancement of organic materials, the introduction of inorganic nanowires is effective due to the percolation effect. In this study, we synthesized Cu2Se NWs by the photoreduction method and prepared poly(3,4-ethylenedioxythiophene):poly (styrene sulfonate) (PEDOT:PSS) thin films containing Cu2Se NWs by spin-coating PEDOT:PSS and Cu2Se NWs alternatively. The composite films exhibited a drastic increase in electrical conductivity at more than 40 wt % Cu2Se, and the Cu2Se amount threshold was in good agreement with surface structures as observed by a scanning electron microscope. This indicates that the percolation effect of connected Cu2Se NWs brought high electrical conductivity. As a result, the composite thin films exhibited a higher power factor than the PEDOT:PSS film. This power factor enhancement by the percolation effect would be expected to contribute to the development of thermoelectric performance enhancement for organic materials.
Masaya Miyagawa, Kengo Nishino, Akane Shibusawa, Hitomi Kotake, and Hideki Tanaka
Plasmonic photoluminescence of Cu nanoparticles was observed in colloidal dispersion by using nanosheets as a support, where acridine orange was adsorbed as an energy donor. The energy transfer took place efficiently followed by the plasmonic photoluminescence, which was enhanced other Cu nanoparticles located nearby on the nanosheet.
Masaya Miyagawa, Yoko Ikeyama, Hitomi Kotake, Toshiki Maeda, and Hideki Tanaka
Green degradation method of Cu nanoparticles was investigated by stepwise oxidation with air and carboxylic acid. The Cu nanoparticles were synthesized directly on kaolinite, one of the layered clay minerals. By the air exposure, the Cu nanoparticles formed Cu2O shell, which was oxidized to Cu2+ by the carboxylic acid. Degradation rate was calculated by using the absorption band of Cu2+. As a result, the Cu nanoparticles were found to be degraded almost completely regardless of valence of the carboxylic acids, while the proton in the carboxy group was significant in the oxidation of Cu2O to Cu2+. Kengo Nishio, Masaya Miyagawa, and Hideki Tanaka
Photoluminescence (PL) of metal nanoparticles (NPs) has historically been investigated with their films, where PL is enhanced by surface plasmon resonance of the NPs themselves. In contrast, it is difficult to observe the PL in colloidal dispersion probably due to too long interparticle distance. In the present study, we observed the PL by introducing Forster resonance energy transfer from acridine orange adsorbed on saponite nanosheets, where Cu NPs were synthesized directly. In the presence of the Cu NPs, the PL spectra were dramatically changed. The PL of the Cu NPs was confirmed by temperature dependency of the PL spectra. Masaya Miyaga, Miharu Usui, Yu Imura, Shota Kuwahara, Toshiki Sugai and Hideki Tanaka
Here, we report a synthesis of Cu nanocubes by photoreduction of CuSO4. Because synthetic saponite (one of the layered clay minerals) was used as the adsorbent, the nanocubes contained no capping agents or protectants, and the disproportionation reaction of Cu2O with H2SO4 was found to be the key for morphological control. Masaya Miyaga, Akane Shibusawa, Kaho Maeda, Akiyoshi Tashiro, Toshiki Sugai and Hideki Tanaka
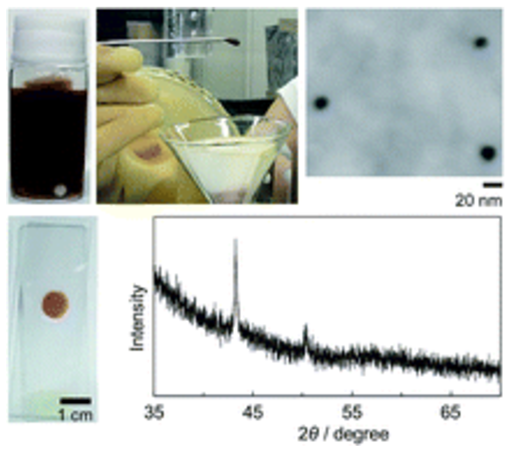
Cu nanoparticles have attracted much attention due to their optical, catalytic, and electrical properties. Their syntheses, however, have required hazardous reducing agents. In addition, they are easily oxidized, which destroys their properties. It has also not been possible to control the diameter and the dispersibility for further applications in functional materials. To solve these problems, we have recently synthesized oxide-free Cu nanoparticles adsorbed on kaolinite, a non-dispersive layered clay, by environmentally friendly photoreduction. Focusing on the layer charge of the clay, we report diameterand dispersibility-controlled Cu nanoparticles on saponite, a dispersive clay, in the present study. The obtained oxide-free Cu nanoparticles were stable under N2 atmosphere, while they exhibited a fast back reaction to Cu2+ upon exposure to fresh air. The diameter of the Cu nanoparticles could be controlled
simply with [Cu2+], because Cu2+ was adsorbed on saponite before the photoreduction. The dispersibility of the Cu nanoparticles was controlled without changing the diameter using the size of saponite aggregates: these were stably dispersed at low [Cu2+], and flocculated at high [Cu2+]. The flocculates could be collected by filtration and the obtained viscous paste was useful for preparing a film with the optical properties of the Cu nanoparticles without aggregation. Therefore, the present study revealed that combining the adsorption of Cu2+ on saponite with photoreduction is a novel and environmentally friendly synthetic method for controlling the diameter and the dispersibility of Cu nanoparticles, and it has the potential to further their applications in functional materials Masaya Miyagawa, Takuya Aoki, Ryoya Seki, Akane Shibusawa, Hideki Tanaka
Truffle-shaped Cu nanoparticles (NPs) were synthesized by photoreduction of copper acetate solution in the presence of TiO2. X-ray diffraction measurement revealed that the obtained NPs were composed of pure Cu without oxides, which was notable because the photoirradiation was performed under atmospheric conditions, where the Cu NPs are easily oxidized. The Cu NPs were dispersed stably without protectants because the TiO2 NPs adsorbed on the Cu NPs were dispersed, probably due to their electric repulsion.
Masaya Miyagawa, Mari Yonemura, Hideki Tanaka
Cu nanoparticle (NP) film has attracted much attention due to its high electric conductivity. In the present study, we prepared a Cu NP film on a TiO2-coated substrate by photoreduction of copper acetate solution. The obtained film showed high electric conductivity and metallic luster by the successive deposition of Cu NP. Moreover, the film was decomposed on exposure to fresh air, and its decomposition reaction mechanisms were proposed. Hence, we concluded that the obtained lustrous film was composed of Cu NP, even though its physical properties was similar to bulk copper.
Masaya Miyagawa, Toshiki Maeda, Ryo Tokuda, Akane Shibusawa, Takuya Aoki, Kazu Okumura and Hideki Tanaka
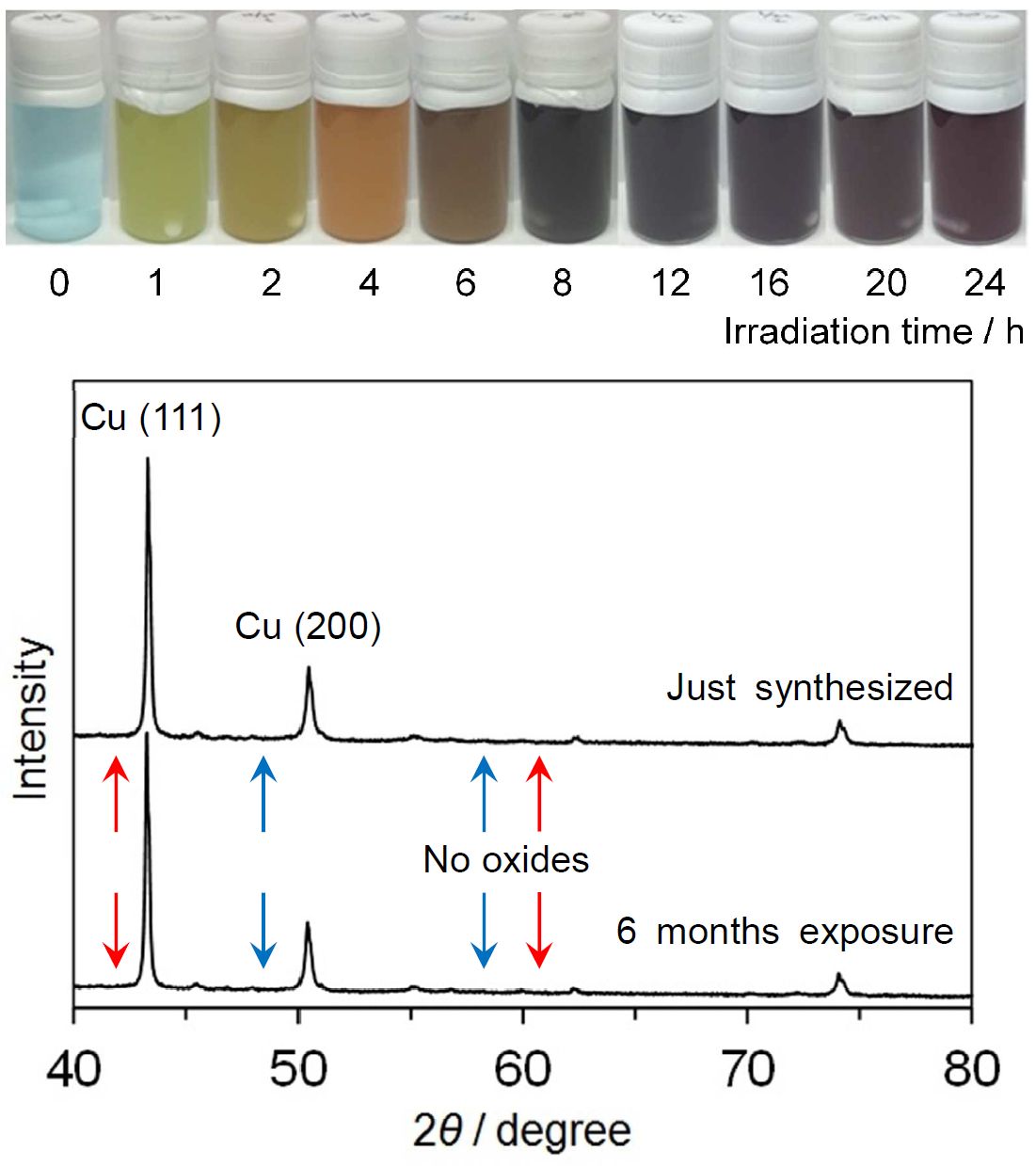
Cu nanoparticles have attracted much attention as an alternative to precious metal Au- and Pt-based materials. However, they have a problem with stability and get oxidized easily. Moreover, their synthesis is carried out with hazardous chemicals and in conditions, which is not environmentally friendly. Thus, both improvement in oxidation resistance and establishment of a novel synthesis method under mild conditions are desired for further applications. To solve these problems, we synthesized Cu nanoparticles from copper(II) acetate by photoreduction at room temperature in the presence of kaolinite, a layered clay mineral with high gas barrier properties. We obtained disk-like metallic Cu nanoparticles adsorbed on kaolinite without oxides. The nanoparticles showed high oxidation resistance in the solid phase. The powder sample was stable and did not get oxidized at all even after 6 months on exposure to fresh air in spite of no apparent protectants, which was revealed by infrared spectroscopy. Namely, the obtained Cu nanoparticles showed a precious metal-like property. On the other hand, the as-prepared Cu nanoparticles still showed the reactivity with fresh air in the suspension. This indicates that the adsorption on kaolinite did not spoil the reactivity of the Cu nanoparticles. Therefore, the present study revealed that the combination of photoreduction and addition of a layered clay mineral would be a promising environmentally friendly way to synthesize oxidation?resistant metal nanoparticles. H Tanaka, T Aoki, M Yonemura, M Miyagawa and K Okumura
We examined the synthesis of copper nanoparticles by photoreduction, and characterized them using optical spectroscopy, XAFS measurement, and electron microscopy. Ethanol solution of copper acetate with TiO2 nanoparticles was photoirradated. Optical absorption observation indicated that copper nanoparticles were formed in the solution. XAFS measurement indicated that the nanoparticles were metallic, not oxidized. Electron microscopy observation exhibited that the nanoparticles kept metallic even under exposure to air.
Naoki Nishida, Yasuhiro Kojima,Hideki Tanaka Chiroptically active Ag triangular nanoplates were synthesized by the ligand-exchange reaction of achiral Ag nanoplates with chiral D- or L-penicillamine molecules. Analysis of the chiroptical properties of the synthesized nanoplates by circular dichroism spectroscopy revealed intense Cotton effects, with wavelengths that corresponded to those of the surface plasmon resonances for the nanoplates. Enantiomeric excess and pH dependence of these effects were also measured to explore the origin of the chiroptical activity of the nanoplates.
Naoki Nishida, Akira Miyashita,Tatsuya Tsukuda, Hideki Tanaka Copper nanoparticles (Cu NPs) were produced through photoreduction of a mixture of copper acetate solution and carbon nanotubes (CNTs) at room temperature. The diameter of the NPs could be controlled by the photoirradiation time. HRTEM and XRD measurements revealed that the NPs were composed of a metallic Cu core and a Cu2O shell that acts as a passive layer. Naoki Nishida, Yasuhiro Kojima,Hideki Tanaka Chiral Ag:SG (glutathione) triangular nanoplates were synthesized by substitution reaction of achiral Ag:poly(vinylpyrrolidone) (PVP) nanoplates with glutathione free GSH molecules. Analysis of the chiroptical properties of the synthesized nanoplates using a circular dichroism spectrometer revealed characteristic Cotton effects with wavelengths that corresponded well to those of plasmon resonances for Ag nanoplates. X-ray photoelectron spectrometry analysis of their chemical compositions revealed that complete substitution of PVP molecules with GSH molecules proceeds on the nanoplates.
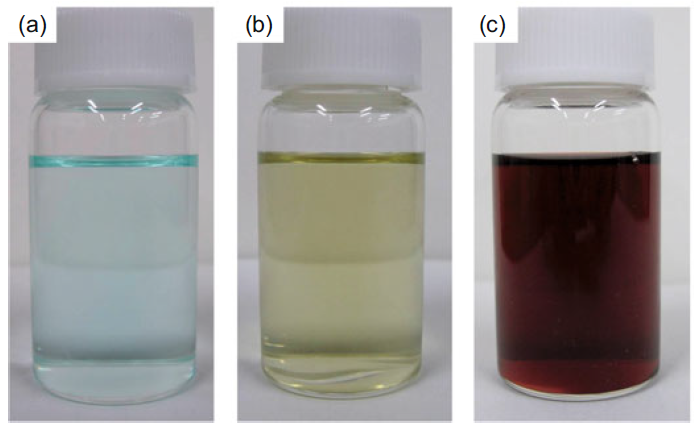
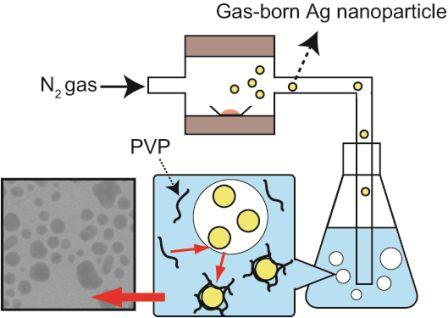
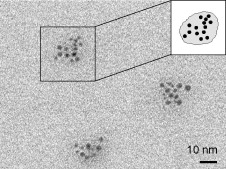
Naoki Nishida, Akira Miyashita, Naomi Hashimoto, Haruno Murayama, Hideki, Tanaka Copper nanoparticles have attracted much attention because of their low cost, and because their use can contribute toward the sustainability of metal resources. In this study, copper nanoparticles were synthesized by the photoirradiation of copper acetate solution at room temperature. The diameter and chemical composition of the obtained copper nanoparticles were analyzed using Field-emission scanning electron microscope (FE-SEM) spectrophotometer and an X-ray photoelectron spectrometer. Well-dispersed copper nanoparticles with `5 nm in diameter were observed in the solution. On the other hand, when the nanoparticle solution was exposed to fresh air, nanoparticles were not observed in the solution. Furthermore, the copper nanoparticles were recovered from a solution of decomposed nanoparticles by re-photoirradiation. Naomi Hashimoto, Naoki Nishida, Haruno Murayama, Hideki Tanaka We demonstrate that gas-born nanoparticles can be transported into solution successfully. Our results show that Ag nanoparticles produced in gas phase are introduced into poly(vinylpyrrolidone) solution without structural changes such as nanoparticle aggregation. It is also shown that Ag nanoparticles in solution show surface plasmon resonance, suggesting that the optical properties of nanoparticles are not impaired by the transport from gas to liquid. Hideki Tanaka, Futoshi Maeda We demonstrate production of nanoparticleclusters, namely clusters composed of nanoparticles, using a combination of surface-active Ag nanoparticles and surface-inactive C60 nanoparticles. The producedclusters, after diameter-selection by a differential mobility analyzer, were analyzed by field-emission scanning electronic microscopy and X-ray photoelectron spectroscopy. The produced clusters are found to be composed of outer surface-deposited Ag nanoparticles with `3 nm diameters that are distinctly isolated from one another and with an inner core composed of C60 molecules. The number of constituent Ag nanoparticles is adjustable by selection of the diameters of the clusters. Haruno Murayama, Naomi Hashimoto, Hideki Tanaka Ag triangular nanoplates were synthesized by photoirradiation to AgNO3 ethanol solution in the presence of polyvinylpyrrolidone (PVP) under anaerobic conditions at room temperature. High-resolution SEM and TEM observations revealed that thin Ag equilateral triangularnanoplates, each of which was a single crystal with sharp corners and flat faces presented by {1 1 1} planes, were produced. Analyses of XAFS and XPS have shown that these nanoplates are protected by coordination of O atoms in PVP molecules, not being oxidized as Ag2O. The nanoplates are inherently stable, though they were easily truncated and dissolved if mixed with oxygen or water. Haruno Murayama, Naomi Hashimoto, Hideki Tanaka We examined to establish the simple synthesis method of Ag triangular nanoplates in order to elucidate their growth process. Ethanol solution of AgNO3 was photoirradiated without reduction agents except in the presence of PVP, poly(vinil-pyrrolidone). Electron microscope observations revealed that the shape of the product was Ag triangular nanoplates with sharp corners and a flat face. In situ XAFS and optical absorption measurements suggested that the Ag triangular nanoplates were photoinduced-converted from Ag spherical nanoparticles via Ag truncated triangular nanoplates. Masaharu Shigeyasu, Haruno Murayama, Hideki Tanaka Production of nanoparticlescomposed of ionicliquid [C4mpyrr][NTf2], N-butyl-N-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide, was demonstrated by a gas aggregation technique under low pressure. The diameter spectra were measured by a differential mobility analyzer (DMA) and were dependent on the heating temperature. Photoelectron spectra for the nanoparticles diameter-selected at 10 nm were also measured by X-ray photoelectron spectroscopy (XPS), and their chemical composition and structure were identified by comparison with photoelectron spectra for neat ionicliquid. The form and surface structure of the nanoparticles deposited onto an Au-coated Si substrate were also determined. Naomi Hashimoto, Haruno Murayama, Hideki Tanaka Ag-containing C60 nanoparticles were produced from Ag vapor interacting with C60 nanoparticles formed by C60 vapor. The particle diameter of the produced nanoparticles was measured using a differential mobility analyzer (DMA). The particle diameter dependence on the Ag vapor temperature was also measured using the DMA. The chemical composition of the produced nanoparticles was investigated by X-ray photoelectron spectroscopy. The experimental results indicated that the C60 nanoparticles worked prominently as a supporting nanomaterial for the Ag nanoparticles.
Nanoscale Adv., 2024, 6, 3299-3305
Catalytic Activity of Nonaggregating Cu Nanoparticles Supported in Pores of Zeolite for Aerobic Oxidation of Benzyl Alcohol
ACS Omega 2024, 9, 970-976
Plasmonic Heating of Copper Nanoparticles with Thermoresponsive Polymers
Chem. Lett. 2023, 52, 7, 582-585
Thermoelectric properties of PEDOT:PSS containing connected copper selenide nanowires synthesized by photoreduction method
ACS Omega 2022, 7, 36, 32101-32107
Plasmonic Photoluminescence of Cu Nanoparticle Realized by Molecular Optical Antenna Designed on Nanosheets
Chem. Lett. 2022, 51, 500-503
Environmental-friendly degradation of clay-hybridized Cu nanoparticles by carboxylic acids
Chemical Physics Letters 753 (2020) 137615
PHOTOLUMINESCENT Cu NANOPARTICLES INDUCED BY ENERGY TRANSFER ON SAPONITE NANOSHEET
Clay Sciene 23, 67-71 (2019)
Aqueous synthesis of protectant-free copper nanocubes by a disproportionation reaction of Cu2O on synthetic saponite
Chem. Commun., 54 (2018) 5854
Diameter-controlled Cu nanoparticles on saponite and preparation of film by using spontaneous phase separation
RSC Adv., 7 (2017) 41896
Synthesis of Truffle-shaped Oxide-free Cu Nanoparticles under Atmospheric Conditions with the Aid of Photocatalytic TiO2
Chem. Lett. 46 (2017) 1403

Lustrous copper nanoparticle film: Photodeposition with high quantum yield and electric conductivity
Chem. Phys. Lett. 655 (2016) 95
Precious metal-like oxide-free copper nanoparticles: high oxidation resistance and geometric structure
RSC Adv. 6 (2016) 104560
Oxidation-resistive copper nanoparticles: photoreduction synthesis and their oxidation state measurements by XAFS and HRTEM
J. Phys. Conf. Ser. 712 (2016) 012120
Intense Plasmon-induced Cotton Effects in Colloidal Silver Triangular Nanoplates Synthesized by a Ligand-exchange Process
Chem. Lett. 43(2014)1227
Production of Oxidation-resistant Copper Nanoparticles on Carbon Nanotubes by Photoreduction
Chem. Lett. 42(2013)168
Chiral glutathione-protected Ag triangular nanoplates synthesized by protectant-substitution reaction-chiroptical and surface structure analysis
Chem. Lett. 41(2012)926
Regenerative synthesis of copper nanoparticles by photoirradiation
Eur. Phys. J. D 63(2011)307
Stable Transport of Gas-born Ag Nanoparticles into Liquid Phase Mediated by Poly (vinylpyrrolidone) Molecules
Chem. Lett. 40(2011)144
Number-adjustablenanoparticleclusters realized by a combination of Ag and C60 nanoparticles in the gas phase
Chemical Physics Letters 484(2009)37
Ag triangular nanoplates synthesized by photo-induced reduction: Structureanalysis and stability
Chemical Physics Letters 482(2009)291
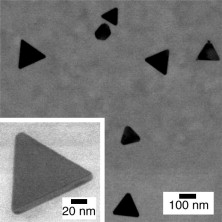
Growth process of Ag triangular nanoplates observed by in situ XAFS
J. Phys. Conf. Ser. 190(2009)012132
Production of nanoparticlescomposed of ionicliquid [C4mpyrr][NTf2] and their chemical identification by diameter analysis and X-ray photoelectron spectroscopy
Chemical Physics Letters@463(2008)373
Production of Small Ag-Containing C60 Nanoparticles under Atmospheric Condition
Jpn. J. Appl. Phys. 47(2008)4777